Voice detects. Sound restores. Together they create an adaptive system for brain health—just as continuous monitoring transformed diabetes, continuous feedback will transform cognitive care.
The Closed-Loop Revolution: Why Continuous Feedback Changes Everything
Millions of people with diabetes now wear continuous glucose monitors—small sensors that track blood sugar every few minutes, automatically alerting their insulin pumps to adjust dosing in real time. This closed-loop system has transformed diabetes management. Compared to manual insulin injections based on periodic finger-stick measurements, hybrid closed-loop systems increase time in target glucose range from 50-60% to 70-80%, while reducing dangerous hypoglycemia by 40-60% (Tauschmann & Hovorka, 2018). The same medication delivers fundamentally different outcomes when paired with continuous measurement and automated response.
The principle is elegantly simple: measure continuously, intervene based on what you measure, reassess to confirm it worked, refine based on results. This closed loop—where measurement directly informs intervention, creating ongoing optimization—has revolutionized treatment across medicine.
Brain health demands this same transformation. The Triple Brain Epidemic—mental illness affecting 60 million Americans annually, cognitive decline costing $1 trillion in care with projected increases as populations age (Brookmeyer et al., 2007; Alzheimer's Association, 2025), and digital overload fragmenting attention for 70% of workers—requires integrated solutions (NIMH, 2024; APA, 2023). Yet our response remains fundamentally fragmented.
"Why Voice", part 1 of this series, established that we lack accessible, continuous measurement. Traditional cognitive tests detect mild cognitive impairment with only 65% sensitivity, missing the very stage when intervention works (Mitchell, 2009; Arevalo-Rodriguez et al., 2015). Mental health conditions are diagnosed only after symptoms force clinical visits, typically years into progression, with systemic barriers limiting timely access to psychiatric care even in well-resourced systems (Kurdyak et al., 2017; Anderson et al., 2019; Chisholm et al., 2016). Digital overload has no diagnostic framework at all, despite emerging evidence of its impact on attention and cognitive load (Zhang et al., 2024). When measurement does occur, it's episodic—quarterly appointments capturing single snapshots while missing the daily fluctuations that matter most.
"Sound as Medicine", part 2 of this series, demonstrated that we have effective interventions. Sound-based therapies modulate neural oscillations, reduce stress biomarkers, and enhance sleep architecture through validated mechanisms. But these interventions are delivered generically, without biomarker feedback to guide personalization, timing, or dosage optimization.
The fundamental problem: measurement and intervention remain disconnected across healthcare.
Antidepressants are prescribed without continuous biomarkers revealing early non-response. Most psychotherapy proceeds without systematic measurement between sessions—while measurement-based care protocols exist and improve outcomes, fewer than 20% of therapists use them consistently due to time constraints, lack of reimbursement, and reliance on self-reported scales that add administrative burden without providing objective data (Lewis et al., 2019; Hatfield & Ogles, 2007). Cognitive decline treatments work only during narrow windows, yet diagnosis happens years too late, and even when detected early, geographic shortfalls of dementia specialists mean many cannot access timely care (Liu et al., 2024; RAND Corporation, 2017). Lifestyle interventions are recommended without feedback on whether they're improving brain health markers. Users receive either episodic measurement or generic intervention, never the continuous feedback loop that makes diabetes management work.
This separation—not merely absence—prevents optimization and leaves millions declining while validated solutions exist in isolated silos.
What Closed-Loop Systems Mean for Brain Health
In neuroscience and medicine, closed-loop systems integrate three elements: continuous measurement of physiological state, interventions delivered based on that measurement, and reassessment that enables ongoing optimization. The defining characteristic: stimulation is delivered when certain conditions are met, and parameters can be adjusted dynamically (Ramasubbu et al., 2018; Parastarfeizabadi & Kouzani, 2017).
In medical contexts, "closed loop" ranges from fully automated systems—like insulin pumps that adjust dosing in real time without human input—to measurement-based care protocols where clinicians receive continuous data that informs treatment adjustments. Vibes AI's platform will operate across this spectrum: certain interventions (like delivering calm-inducing frequencies when voice biomarkers detect acute stress) can respond automatically within minutes, while others (like identifying optimal gamma frequencies for an individual) involve iterative refinement over weeks as the system learns which parameters produce measurable cognitive improvements.
The clinical evidence is compelling across domains. Responsive neurostimulation for epilepsy delivers therapy only when neural signatures predict imminent seizures, achieving over 40% seizure reduction while minimizing side effects by restricting stimulation to moments of actual need (Morrell, 2011; Bergey et al., 2015). Traditional continuous medication causes cognitive side effects while preventing only some seizures. Closed-loop responsive systems outperform by matching intervention precisely to physiological state.
In mental health, measurement-based care—where treatment adjusts based on continuous symptom tracking—consistently enhances outcomes. Meta-analyses show that adding progress feedback to psychological interventions improves effectiveness, with particularly strong benefits for patients not progressing well on initial approaches (Lambert et al., 2018; Lewis et al., 2019). The mechanisms illuminate why: feedback identifies deteriorating cases that clinicians often miss without objective data, corrects providers' tendency to overestimate treatment success, and enables timely adjustments within days rather than waiting months between assessments (Lambert et al., 2018; Scott & Lewis, 2015). Yet measurement-based care remains underutilized, and when implemented, relies on subjective symptom reports rather than objective biomarkers.
Adaptive clinical trial designs operationalize the same principle in research. By modifying protocols based on interim data, these trials identify effective treatments faster, reduce participant exposure to ineffective interventions, and improve statistical power through enrichment strategies—all while maintaining scientific rigor (Berry et al., 2010; Bhatt & Mehta, 2016).
Digital therapeutics for chronic diseases demonstrate that closed-loop principles extend beyond clinical hardware to consumer devices, with just-in-time adaptive interventions delivering personalized support based on real-time data (Nahum-Shani et al., 2018). Asthma platforms tracking inhaler use and delivering real-time feedback improve medication adherence and disease control without requiring specialized clinical infrastructure (Chan et al., 2023). Hypertension management apps that adjust recommendations based on continuous blood pressure monitoring achieve better outcomes than static treatment protocols (Zietzer et al., 2025).
The pattern holds universally: dynamic systems that sense, respond, and learn outperform static protocols by orders of magnitude (Parastarfeizabadi & Kouzani, 2017). This advantage is particularly crucial for brain health, where cognitive and emotional states fluctuate throughout the day based on sleep quality, stress levels, social interactions, and countless other factors.
Voice + Sound: The Natural Closed Loop for Brain Health
Brain health presents unique requirements for closed-loop systems. Unlike diabetes where glucose levels change over hours, cognitive and emotional states fluctuate throughout the day—stress spikes during meetings, attention fragments after poor sleep, mood shifts with circadian rhythms. Effective intervention must match this temporal complexity.
Voice and sound operate on compatible timescales: voice captures state changes within minutes through natural speech, while therapeutic audio delivers neural modulation that takes effect within 20 minutes through passive listening. This temporal alignment, combined with the accessibility of consumer devices, makes voice and sound the natural foundation for continuous brain health optimization.
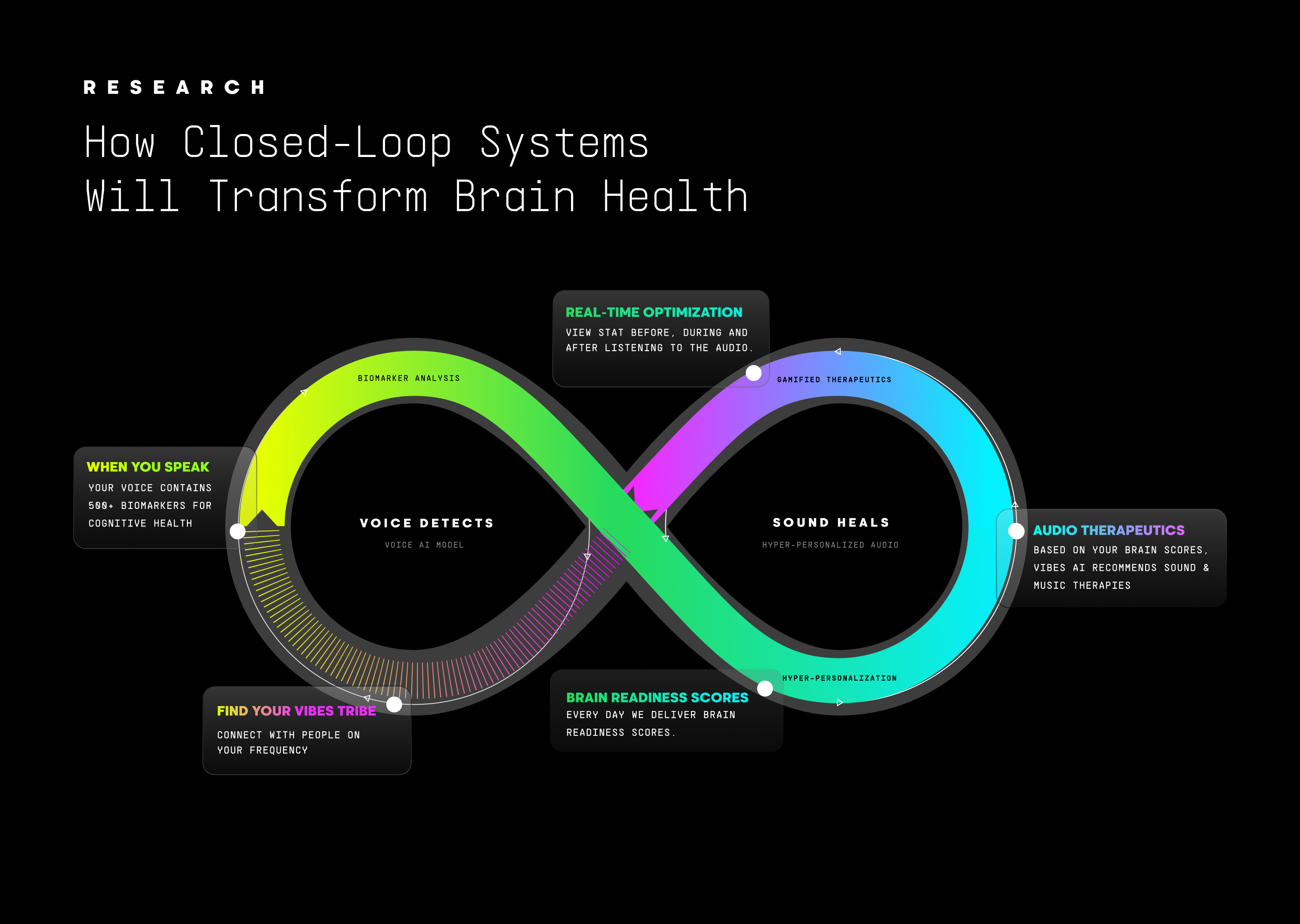
Part 1, "Why Voice", established voice as a comprehensive biomarker, detecting changes years before traditional methods. Part 2, "Sound as Medicine", demonstrated sound's validated neurophysiological mechanisms—gamma entrainment, rhythmic motor system activation (Thaut et al., 2015), vagal activation, sleep optimization. Together, they form a continuous cycle: Voice detects state → Sound delivers personalized intervention → Voice reassesses response → System refines approach.

How Closed-Loop Systems Will Transform Brain Health
Could users assemble their own closed loop by combining separate voice-tracking and sound therapy apps? Technically possible—but practically, this fails for the same reason that manually logging blood sugar and calculating insulin doses failed for diabetes management before continuous glucose monitors. Closed loops work because they eliminate human intervention friction: the measurement-intervention-optimization cycle happens automatically, continuously, without requiring users to interpret data, select appropriate interventions, or remember to reassess outcomes. Cognitive load—the very thing declining in populations who need this most—becomes the barrier. Integrated systems succeed because they remove the burden of orchestration, allowing the technology to work passively while users focus on living.
The Vision: Closed-Loop Brain Health Across the Lifespan
The integrated Vibes AI platform we're building will bring this closed-loop vision to life across the full spectrum of the Triple Brain Epidemic through two complementary pathways: individual closed loops within the platform and integration with clinical care systems.
Individual Closed Loops: Daily voice biomarkers reveal patterns invisible to traditional assessments. Personalized Restorative Audio responds dynamically based on detected state. Continuous optimization completes the loop by assessing whether interventions worked.
Voice captures shifts through acoustic features (fundamental frequency, jitter, shimmer, speech rate), linguistic characteristics (lexical complexity, semantic content, fluency), and respiratory patterns during speech produced through laryngeal motor cortex coordination (Simonyan & Horwitz, 2011)—all extracted passively during natural daily interactions (Alhanai et al., 2017; Lin et al., 2020; Mahon & Lachman, 2022; Ding et al., 2024). When voice biomarkers detect elevated stress, the platform delivers vagal-activating low-frequency tones to restore parasympathetic balance, measurably increasing heart rate variability within 20 minutes (Punkanen & Ala-Ruona, 2012; Du et al., 2022; Mojtabavi et al., 2020). When sleep quality declines, closed-loop acoustic stimulation enhances slow-wave oscillations, improving memory consolidation by 40% and next-day cognitive performance (Ngo et al., 2013; Papalambros et al., 2017). When attention fragments from digital overload, gamma-frequency entrainment at 40 Hz restores focused cognitive states while supporting cellular clearance mechanisms relevant to long-term brain health (Iaccarino et al., 2016; Martorell et al., 2019; Nozaradan et al., 2011; Ruhnau et al., 2016), with sound-based therapies also reducing systemic inflammation through validated psychoneuroimmunological pathways (Fancourt et al., 2014).
This sleep-dependent memory consolidation mechanism also explains why acoustic optimization during midlife may prevent later-life dementia. During deep sleep, the glymphatic system clears metabolic waste including amyloid-beta and tau proteins (Xie et al., 2013). Enhancing slow-wave sleep architecture throughout the 40s-60s—the decades when natural sleep quality declines but before amyloid pathology becomes irreversible—creates a continuous neuroprotective effect that compounds over years.
Subsequent voice samples reveal whether interventions produced intended effects, enabling iterative refinement—if certain audio compositions consistently improve outcomes for a specific user, the system learns to prioritize those approaches. Biomarker-driven therapeutic adjustment dramatically shortens the time to optimal therapy compared to episodic assessment (Ramos-Lima et al., 2020). The platform becomes smarter over time, continuously refining its understanding of what works for each individual.
Clinical Integration: Voice biomarkers create measurement infrastructure that transforms how any intervention is delivered. If you opt-in, continuous, accessible monitoring between episodic clinical visits means your doctor sees cognitive trajectories over 90 days rather than quarterly snapshots. Your therapist could receive alerts when mental health markers deteriorate between sessions. You gain visibility into patterns invisible to traditional assessment—the daily fluctuations that determine whether interventions are working.
Together, voice and sound create a closed loop within the platform while feeding valuable longitudinal data to the broader healthcare system. The result: fewer people progressing from prevention to management to crisis, and better outcomes for those who do require clinical care.
This dual-pathway model serves the full spectrum of need:
Proactive Prevention: Digital Overload Before It Takes Hold
Maya, 24, works in social media marketing. She spends 10+ hours daily on screens and recently noticed trouble focusing during evening reading. Her morning voice check reveals acoustic markers of cognitive load accumulating: slightly increased speech rate, reduced prosodic variation, linguistic patterns suggesting sustained stress. The platform delivers gamma-frequency entrainment while she prepares for the next day's creative work to support attention networks, and recommends evening soundscapes that facilitate cognitive recovery. Within weeks, her voice biomarkers stabilize. She's intervening in the 20-year window before digital overload becomes chronic cognitive impairment—prevention at its most powerful.
Managing Present Challenges: The Mid-Career Convergence
Sarah, 42, is a software engineer living in the collision zone where biology meets modern life. Perimenopause has turned sleep into a negotiation her body keeps losing. She wakes at 3 AM, mind racing through code reviews and tween logistics, her heart pounding with a stress response she can't shut off. By morning voice check, her speech patterns tell the story her exhausted smile tries to hide: elevated pitch variability, compressed pauses, the acoustic signature of a nervous system running on override.
The platform delivers personalized low-frequency vibroacoustic tones during her commute, targeting vagal activation. By midday, autonomic balance improves, but cognitive fatigue emerges—longer pauses, reduced prosodic complexity signal declining executive function under compounding demands. The system adapts, delivering Restorative Audio during her afternoon focus block. Evening voice checks show cognitive metrics stabilizing; the platform recommends sleep-preparation soundscapes calibrated to her individual architecture and hormonal patterns.
Over weeks, the system learns her cycles: certain weeks show heightened stress markers correlating with hormonal shifts, Friday afternoons reveal cumulative family and work fatigue, her optimal gamma frequency is 42 Hz rather than standard 40 Hz, reflecting individual differences in neural oscillation patterns (Cecere et al., 2015). The platform anticipates needs and delivers increasingly precise support for this complex life stage.
Sarah also shares her longitudinal voice data with her therapist through the platform's clinical integration features. When her biomarkers show a concerning downward trend mid-cycle, her therapist receives an alert and schedules an additional session—catching deterioration that would have gone unnoticed for weeks under the traditional monthly appointment model. The therapeutic audio provides immediate support between sessions while her therapist adjusts cognitive-behavioral interventions based on objective data rather than Sarah's subjective recall of symptoms.
Supporting Loved Ones: Managing Alzheimer's at Late Stages
Sam, 58, manages care for her mother who has severe Alzheimer's disease. At this stage, verbal communication is greatly diminished, making voice biomarkers infeasible. Instead, Vibes’ platform draws from alternative data streams: sleep tracking from her mother's wearable shows fragmented nights with frequent wakings, movement sensors detect increasing nighttime agitation, and Sam's caregiver assessments through the app document behavioral patterns.
Six months ago, these integrated biomarkers showed her mother's symptoms escalating toward crisis—the data painted a clear picture of declining sleep quality and mounting agitation that threatened institutional placement. Today, the closed loop works through multi-modal monitoring: the platform synthesizes sleep data, movement patterns, and caregiver observations to generate personalized audio protocols. A precisely engineered Restorative Audio session plays through the family's smart speaker each morning dynamically calibrated based on last night's sleep quality and agitation levels.
Within minutes, Sam sees the difference. Her mother is calmer, more alert. Sam's own stress levels ease as the household regains balance. Follow-up data shows measurably improved sleep patterns and reduced agitation markers. What once felt like inevitable decline now feels manageable, supported by sound science and integrated biomarkers delivered through everyday devices. Meanwhile, Sam shares this longitudinal data with her mother's neurologist, who uses the objective behavioral tracking to optimize medication timing and dosing—decisions previously made based on Sam's subjective reports from brief office visits.
These scenarios demonstrate the dual-pathway advantage: Maya engages the individual closed loop proactively, maintaining cognitive wellness and never requiring clinical care because intervention prevents digital overload from becoming chronic impairment. Sarah benefits from both pathways—voice biomarkers alert her to declining mental health markers while therapeutic audio provides immediate support, preventing crisis escalation, and her patterns inform her therapist's approach between sessions. Sam's scenario demonstrates full healthcare integration—multi-modal monitoring creates longitudinal data that helps her mother's neurologist optimize medication management while audio reduces behavioral symptoms and delays institutionalization.
The Infrastructure Advantage: An Expandable, Learning System
While voice and sound form the initial closed loop, the platform architecture reflects a fundamental principle from systems neuroscience: the brain itself is multi-modal and adaptive. No single biomarker captures complete cognitive state. No single intervention typically addresses all needs. Brain health infrastructure must mirror the brain's own complexity—integrating multiple streams of information, adapting interventions based on response, and continuously refining its approach.
Multi-modal biomarker integration enhances prediction accuracy. Research demonstrates that combining voice with sleep data from wearables, activity patterns from smartphones, heart rate variability, and other digital phenotypes creates more comprehensive cognitive profiles than any single measure. Statistically independent biomarkers provide incremental improvement in identifying individuals at risk, with integrated scores outperforming single modalities (Finan et al., 2018; O'Connor et al., 2021).
The platform architecture extends beyond direct-to-consumer applications into infrastructure that powers cognitive health across multiple channels. MANTRA voice biomarkers can inform interventions delivered through partner platforms—healthcare systems using our API to detect cognitive decline during routine telehealth visits, corporate wellness programs supporting employee cognitive performance, hardware manufacturers like Oura, Samsung, and Apple integrating cognitive readiness scores into their health ecosystems, or pharmaceutical companies licensing our measurement infrastructure for clinical trials.
Vibes AI maintains the closed loop within our own platform through voice biomarkers and therapeutic audio. Beyond our platform, MANTRA provides the measurement layer that makes any cognitive intervention more effective—whether delivered by clinicians, wellness programs, or other technology partners. The longitudinal voice-cognition dataset we're building becomes the foundation for an ecosystem where cognitive health monitoring is as ubiquitous as heart rate tracking, enabling better outcomes regardless of where intervention occurs.
As new biomarker technologies emerge and additional evidence-based interventions are validated, they can be incorporated into this expanding ecosystem. The infrastructure scales horizontally—more measurement modalities and intervention options—while maintaining the closed-loop architecture that makes continuous optimization possible. This mirrors how the brain itself operates: multiple systems working in parallel, constantly adapting based on feedback, optimizing performance through experience.
From Reactive Care to Adaptive Wellness
The journey through this series reveals a transformation in how we approach brain health. Part 1, "Why Voice", established that voice functions as the fifth vital sign——detecting cognitive and emotional changes years before traditional methods with diagnostic accuracies exceeding 90%. Part 2, "Sound as Medicine", demonstrated that precisely calibrated acoustic stimulation modulates neural oscillations, activates vagal pathways, enhances sleep, and reduces inflammation through validated mechanisms. This piece illuminates why integration creates exponential value: the closed loop transforms both detection and intervention into continuous optimization.
Ancestral traditions recognized sound's power to shift consciousness and facilitate healing. Modern medicine dismissed these practices while treating the brain as inscrutable—a black box observable only after damage appeared. Voice and sound unite ancestral wisdom with rigorous neuroscience. The brain speaks constantly through speech patterns, revealing its state minute by minute. Sound responds directly, modulating the electrical rhythms that generate cognition itself.
The integrated closed-loop platform launches in Q1 2026. Meanwhile, condition-specific Restorative Audio is already delivering measurable outcomes across Apple Podcasts, Spotify, Amazon Music, and more.
Not occasional clinical snapshots followed by generic prescriptions. Not reactive crisis management after irreversible damage. Continuous optimization meeting the brain where it actually operates—in daily conversations, during commutes, throughout sleep cycles—with interventions that require no appointments, no prescriptions, no cognitive effort.
Voice reveals, sound restores, and together they create the first closed-loop system for lifelong brain wellness.
The closed loop is coming—and it changes everything about who can access brain health, when they can access it, and what outcomes become possible when measurement and intervention finally unite.
References
Alhanai, T., Au, R., & Glass, J. (2017). Spoken language biomarkers for detecting cognitive impairment. 2017 IEEE Automatic Speech Recognition and Understanding Workshop (ASRU), 409-416. https://doi.org/10.1109/ASRU.2017.8268965
Alzheimer's Association. (2025). 2025 Alzheimer's Disease Facts and Figures. Alzheimer's & Dementia, 21(5). https://www.alz.org/getmedia/ef8f48f9-ad36-48ea-87f9-b74034635c1e/alzheimers-facts-and-figures.pdf
American Psychological Association. (2023). 2023 Work in America Survey. https://www.apa.org/pubs/reports/work-in-america/2023-workplace-health-well-being
Arevalo-Rodriguez, I., Smailagic, N., Roqué I Figuls, M., Ciapponi, A., Sanchez-Perez, E., Giannakou, A., Pedraza, O. L., Bonfill Cosp, X., & Cullum, S. (2015). Mini-Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews, 2015(3), CD010783. https://doi.org/10.1002/14651858.CD010783.pub2
Bergey, G. K., Morrell, M. J., Mizrahi, E. M., et al. (2015). Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology, 84(8), 810-817. https://doi.org/10.1212/WNL.0000000000001280
Berry, S. M., Carlin, B. P., Lee, J. J., & Muller, P. (2010). Bayesian adaptive methods for clinical trials. CRC Press. https://doi.org/10.1201/EBK1439825488
Bhatt, D. L., & Mehta, C. (2016). Adaptive designs for clinical trials. New England Journal of Medicine, 375(1), 65-74. https://doi.org/10.1056/NEJMra1510061
Brookmeyer, R., Johnson, E., Ziegler-Graham, K., & Arrighi, H. M. (2007). Forecasting the global burden of Alzheimer's disease. Alzheimer's & Dementia, 3(3), 186-191. https://doi.org/10.1016/j.jalz.2007.04.381
Cecere, R., Rees, G., & Romei, V. (2015). Individual differences in alpha frequency drive crossmodal illusory perception. Current Biology, 25(2), 231-235. https://doi.org/10.1016/j.cub.2014.11.034
Chan, A. H., Harrison, J., Black, P. N., Mitchell, E. A., & Foster, J. M. (2015). Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. The journal of allergy and clinical immunology. In practice, 3(3), 335–49.e495. https://doi.org/10.1016/j.jaip.2015.01.024
Chisholm, D., Sweeny, K., Sheehan, P., et al. (2016). Scaling-up treatment of depression and anxiety: A global return on investment analysis. The Lancet Psychiatry, 3(5), 415-424. https://doi.org/10.1016/S2215-0366(16)30024-4
Ding, H., Lister, A., Karjadi, C., et al. (2024). Detection of Mild Cognitive Impairment From Non-Semantic, Acoustic Voice Features: The Framingham Heart Study. JMIR Aging, 7, e55126. https://doi.org/10.2196/55126
Du, J., Shi, P., Fang, F., & Yu, H. (2022). Effect of music intervention on subjective scores, heart rate variability, and prefrontal hemodynamics in patients with chronic pain. Frontiers in Human Neuroscience, 16, 1057290. https://doi.org/10.3389/fnhum.2022.1057290
Fancourt, D., Ockelford, A., & Belai, A. (2014). The psychoneuroimmunological effects of music: A systematic review and a new model. Brain, Behavior, and Immunity, 36, 15-26. https://doi.org/10.1016/j.bbi.2013.10.014
Finan, P. H., Goodin, B. R., & Smith, M. T. (2018). The association of sleep and pain: An update and a path forward. Journal of Pain, 14(12), 1539-1552. https://doi.org/10.1016/j.jpain.2013.08.007
Hatfield, D. R., & Ogles, B. M. (2007). Why some clinicians use outcome measures and others do not. Administration and Policy in Mental Health and Mental Health Services Research, 34(3), 283-291. https://doi.org/10.1007/s10488-006-0110-y
Iaccarino, H. F., Singer, A. C., Martorell, A. J., et al. (2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature, 540(7632), 230-235. https://doi.org/10.1038/nature20587
Anderson, K. K., John-Baptiste, A., MacDougall, A. G., Li, L., Kurdyak, P., & Osuch, E. A. (2019). Access and Health System Impact of an Early Intervention Treatment Program for Emerging Adults with Mood and Anxiety Disorders. Canadian journal of psychiatry. Revue canadienne de psychiatrie, 64(7), 492–500. https://doi.org/10.1177/0706743718809347
Kurdyak, P., Stukel, T. A., Goldbloom, D., Kopp, A., Zagorski, B. M., & Mulsant, B. H. (2014). Universal coverage without universal access: a study of psychiatrist supply and practice patterns in Ontario. Open medicine : a peer-reviewed, independent, open-access journal, 8(3), e87–e99.
Lambert, M. J., Whipple, J. L., & Kleinstäuber, M. (2018). Collecting and delivering progress feedback: A meta-analysis of routine outcome monitoring. Psychotherapy, 55(4), 520-537. https://doi.org/10.1037/pst0000167
Lewis, C. C., Boyd, M., Puspitasari, A., et al. (2019). Implementing measurement-based care in behavioral health: A review. JAMA Psychiatry, 76(3), 324-335. https://doi.org/10.1001/jamapsychiatry.2018.3329
Lin, H., Karjadi, C., Ang, T. F. A., et al. (2020). Identification of digital voice biomarkers for cognitive health. Exploration of Medicine, 1, 406-417. https://doi.org/10.37349/emed.2020.00028
Liu, J. L., Baker, L., Chen, A. Y., & Wang, J. J. (2024). Geographic variation in shortfalls of dementia specialists in the United States. Health Affairs Scholar, 2(7), qxae088. https://doi.org/10.1093/haschl/qxae088
Mahon, E., & Lachman, M. E. (2022). Voice biomarkers as indicators of cognitive changes in middle and later adulthood. Neurobiology of Aging, 119, 22-35. https://doi.org/10.1016/j.neurobiolaging.2022.06.010
Martorell, A. J., Paulson, A. L., Suk, H. J., et al. (2019). Multi-sensory gamma stimulation ameliorates Alzheimer's-associated pathology and improves cognition. Cell, 177(2), 256-271.e22. https://doi.org/10.1016/j.cell.2019.02.014
Mitchell, A. J. (2009). A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. Journal of Psychiatric Research, 43(4), 411-431. https://doi.org/10.1016/j.jpsychires.2008.04.014
Mojtabavi, H., Saghazadeh, A., Valenti, V. E., & Rezaei, N. (2020). Can music influence cardiac autonomic system? A systematic review and narrative synthesis to evaluate its impact on heart rate variability. Complementary Therapies in Clinical Practice, 39, 101162. https://doi.org/10.1016/j.ctcp.2020.101162
Morrell, M. J. (2011). Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology, 77(13), 1295-1304. https://doi.org/10.1212/WNL.0b013e3182302056
Nahum-Shani, I., Smith, S. N., Spring, B. J., et al. (2018). Just-in-time adaptive interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine, 52(6), 446-462. https://doi.org/10.1007/s12160-016-9830-8
National Institute of Mental Health. (2024). Mental illness. https://www.nimh.nih.gov/health/statistics/mental-illness
Ngo, H. V., Martinetz, T., Born, J., & Mölle, M. (2013). Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron, 78(3), 545-553. https://doi.org/10.1016/j.neuron.2013.03.006
Nozaradan, S., Peretz, I., Missal, M., & Mouraux, A. (2011). Tagging the neuronal entrainment to beat and meter. Journal of Neuroscience, 31(28), 10234-10240. https://doi.org/10.1523/JNEUROSCI.0411-11.2011
O'Connor, M. F., Bower, J. E., Cho, H. J., et al. (2021). To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity, 23(7), 887-897. https://doi.org/10.1016/j.bbi.2009.04.005
Papalambros, N. A., Santostasi, G., Malkani, R. G., et al. (2017). Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Frontiers in Human Neuroscience, 11, 109. https://doi.org/10.3389/fnhum.2017.00109
Parastarfeizabadi, M., & Kouzani, A. Z. (2017). Advances in closed-loop deep brain stimulation devices. Journal of NeuroEngineering and Rehabilitation, 14, 79. https://doi.org/10.1186/s12984-017-0295-1
Punkanen, M., & Ala-Ruona, E. (2012). Contemporary vibroacoustic therapy: Perspectives on clinical practice, research, and training. Music and Medicine, 4(3), 128-135. https://doi.org/10.1177/1943862112445324
Ramasubbu, R., Clark, D. L., Golding, S., Dobson, K. S., Mackie, A., Haffenden, A., & Kiss, Z. H. (2020). Long versus short pulse width subcallosal cingulate stimulation for treatment-resistant depression: a randomised, double-blind, crossover trial. The lancet. Psychiatry, 7(1), 29–40. https://doi.org/10.1016/S2215-0366(19)30415-8
Ramos-Lima, L. F., Waikamp, V., Antonelli-Salgado, T., et al. (2020). The use of machine learning techniques in trauma-related disorders: A systematic review. Journal of Psychiatric Research, 121, 159-172. https://doi.org/10.1016/j.jpsychires.2019.12.001
RAND Corporation. (2017). Assessing the Preparedness of the U.S. Health Care System Infrastructure for an Alzheimer's Treatment. https://www.rand.org/pubs/research_reports/RR2272.html
Ruhnau, P., Hauswald, A., & Weisz, N. (2014). Investigating ongoing brain oscillations and their influence on conscious perception - network states and the window to consciousness. Frontiers in psychology, 5, 1230. https://doi.org/10.3389/fpsyg.2014.01230
Scott, K., & Lewis, C. C. (2015). Using measurement-based care to enhance any treatment. Cognitive and Behavioral Practice, 22(1), 49-59. https://doi.org/10.1016/j.cbpra.2014.01.010
Simonyan, K., & Horwitz, B. (2011). Laryngeal motor cortex and control of speech in humans. The Neuroscientist, 17(2), 197-208. https://doi.org/10.1177/1073858410386727
Tauschmann, M., & Hovorka, R. (2018). Technology in the management of type 1 diabetes mellitus -- current status and future prospects. Nature Reviews Endocrinology, 14(8), 464-475. https://doi.org/10.1038/s41574-018-0044-y
Thaut, M. H., McIntosh, G. C., & Hoemberg, V. (2015). Neurobiological foundations of neurologic music therapy: Rhythmic entrainment and the motor system. Frontiers in Psychology, 5, 1185. https://doi.org/10.3389/fpsyg.2014.01185
Xie, L., Kang, H., Xu, Q., et al. (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373-377. https://doi.org/10.1126/science.1241224
Zhang, X., et al. (2024). Information overload in mobile health applications and medical service overuse: A prospective study. Frontiers in Public Health, 12, 1408998. https://doi.org/10.3389/fpubh.2024.1408998
Zietzer, A., Düsing, P., Brokamp, F., Schäfer, S., Stieber, F., Wilhelm, K., Zgouras, D., & Schirmer, S. H. (2025). A Smartphone-Guided Digital Health Application for Hypertension: The Randomized Controlled HELP Trial. Deutsches Arzteblatt international, 122(11), 292–297. https://doi.org/10.3238/arztebl.m2025.0066
About Vibes AI
Vibes AI is a neurotechnology company on a mission to accelerate the world's access to cognitive health & wellness. Founded in 2024, the company uses AI, neuroscience, and ancestral intelligence to create innovative solutions that make cognitive health and enhancement accessible to all. MANTRA, one of the company's flagship products, uses voice biomarker technology to detect early signs of cognitive decline and provide personalized interventions.
No Comments.